Global Vision
World-Class Manufacturing System
◆Achieving Advanced Manufacturing and Quality Control
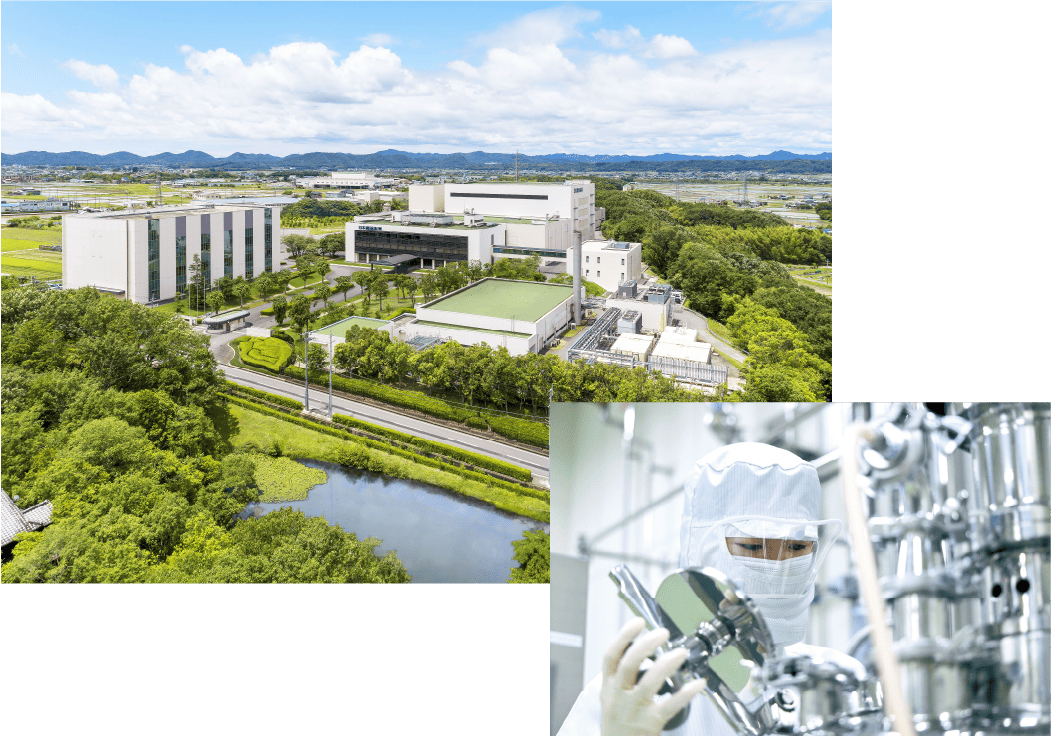


◆Achieving Advanced Manufacturing and Quality Control
At our pharmaceutical manufacturing facilities, we have established a robust system to ensure a stable supply of key products, including Neurotropin, under advanced manufacturing and quality control standards. In compliance with Good Manufacturing Practice (GMP)—the international standard for pharmaceutical manufacturing—we conduct integrated production ranging from drug substances to solid oral dosage forms and sterile injectables. Our manufacturing system has been evaluated as appropriate by regulatory authorities both in Japan and overseas.
In particular, Neurotropin is a formulation derived from biomaterials, and we therefore implement high-level manufacturing and quality control measures to ensure its efficacy and safety.
Furthermore, in order to respond to continuously evolving international regulations and advances in pharmaceutical manufacturing technologies, we actively collect information related to manufacturing and quality control both in Japan and abroad, and continuously work to enhance product quality and safety. We are also promoting digital transformation (DX) initiatives to manage and optimize manufacturing site operations.
In addition, with the aim of becoming a “pharmaceutical manufacturing facility that is friendly to both people and the environment,” we strive to achieve harmony between production activities and environmental stewardship while safeguarding the health of our employees. In particular, we have installed high-performance wastewater treatment facilities and incineration systems to help preserve the surrounding environment.
◆Production of Raw Materials for Drug Substances in China and Vietnam

◆Production of Raw Materials for Drug Substances in China and Vietnam
Through our group companies operating in China and Vietnam, we are engaged in the production of raw materials for drug substances. These operations play an indispensable role in ensuring quality control and a stable supply of our main ethical pharmaceutical products.
Looking ahead, we are also considering pharmaceutical manufacturing activities in China and Vietnam as part of our broader manufacturing strategy.

